The textile industry relies heavily on chemistry to transform raw fibers into finished fabrics with desirable qualities such as color, softness, strength, and durability. Each stage of textile production—from fiber preparation to dyeing and finishing—requires specific chemicals to achieve consistent, high-quality results. Here’s an overview of the most common chemicals used in the textile industry and their functions.
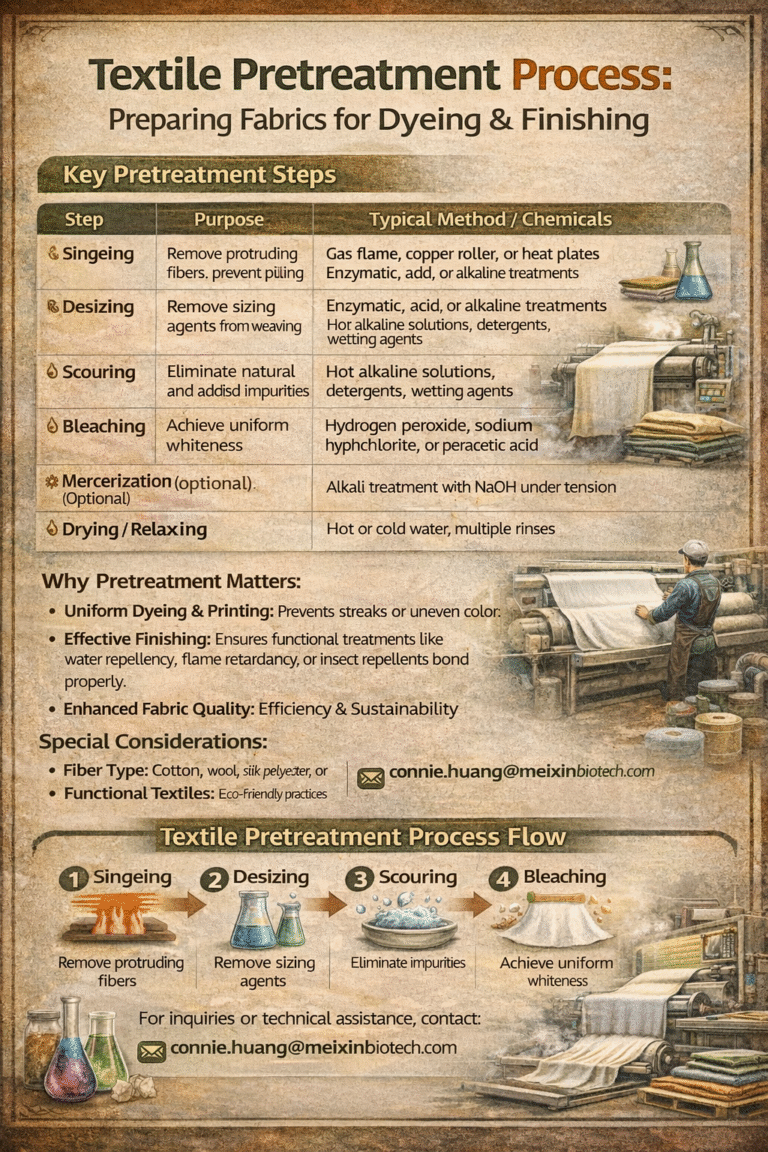
1. Chemicals in Fiber Preparation
Before dyeing or finishing, fibers undergo pretreatment to remove impurities and prepare them for further processing.
- Caustic Soda (Sodium Hydroxide): Used in scouring to remove natural oils, waxes, and impurities from cotton.
- Hydrogen Peroxide: Acts as a bleaching agent to whiten fibers and improve dye uptake.
- Sodium Silicate: Stabilizes hydrogen peroxide during bleaching, ensuring even results.
2. Chemicals in Dyeing
Chemistry gives fabrics their vibrant and lasting colors through different classes of dyes.
- Reactive Dyes: Form strong covalent bonds with cellulose fibers, ensuring bright and wash-fast colors.
- Acid Dyes: Ideal for protein fibers like silk and wool, producing rich and vibrant shades.
- Disperse Dyes: Used for synthetic fibers such as polyester, ensuring uniform coloration.
- Mordants (e.g., Metal Salts): Improve dye fixation and enhance colorfastness.
3. Chemicals in Printing
Printing applies patterns and designs to textiles, and chemistry ensures these designs remain durable.
- Binders: Fix pigments onto fabric surfaces.
- Thickeners: Control the viscosity of printing pastes for sharp and accurate patterns.
- Fixing Agents: Enhance wash and rub fastness of prints.
4. Chemicals in Finishing
Finishing chemicals provide fabrics with functional and aesthetic properties that meet consumer demands.
- Softeners (Silicone or Fatty Acids): Improve fabric feel and comfort.
- Resins (Formaldehyde-based or Modified Alternatives): Provide wrinkle resistance and shrink control.
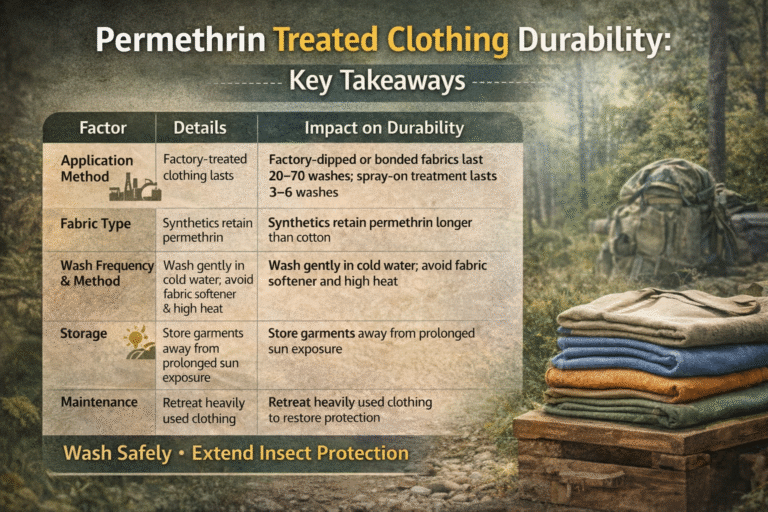
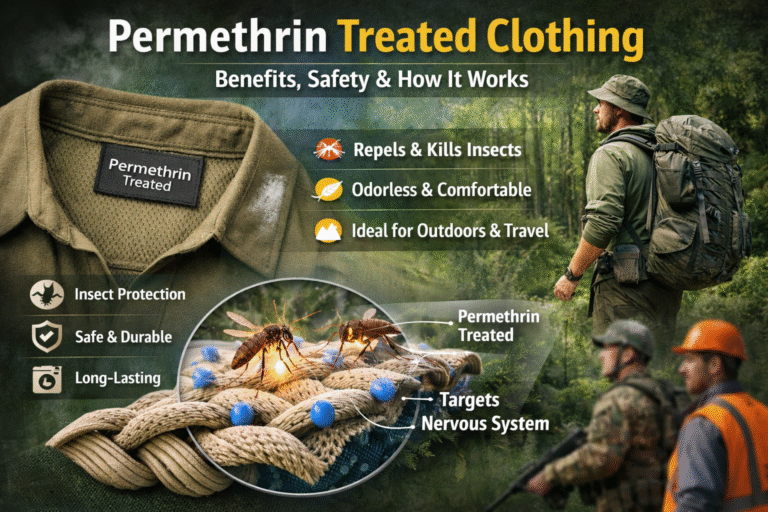
- Flame Retardants (Phosphorus or Nitrogen Compounds): Reduce flammability in protective textiles.
- Water- and Oil-Repellent Agents (Fluorochemicals, Silicones): Protect fabrics from stains and moisture.
- Antimicrobial Agents (Silver Nanoparticles, Quaternary Ammonium Compounds): Prevent bacterial and fungal growth, ideal for medical and sports textiles.
5. Chemicals in Sustainable Textile Processing
Modern textile chemistry is also evolving toward eco-friendly solutions.
- Enzymes: Replace harsh chemicals in scouring, bleaching, and finishing.
- Low-Impact Dyes: Reduce water and energy usage while minimizing pollution.
- Biodegradable Finishing Agents: Ensure textiles are safer for the environment.


Conclusion
The textile industry relies on a wide range of chemicals to produce fabrics that are colorful, durable, comfortable, and functional. From fiber preparation to finishing, chemistry ensures textiles meet the needs of modern consumers while advancing toward sustainability.
For reliable chemical solutions and technical support in textiles, contact Meixin Biotech at connie.huang@meixinbiotech.com.
Textile Auxiliaries Industry Articles
The Role of Chemistry in Textile Finishing Processes
The Science Behind Fabrics: How Chemistry Shapes What We Wear
Why Chemistry is Essential for Modern Textiles
The Role of Chemistry in the Textile Industry: An Overview
How Chemistry Enhances Comfort, Durability, and Style in Fabrics
Understanding Textile Auxiliaries: The Hidden Chemistry Behind Fabric Quality
Most Common Chemicals Used in the Textile Industry and Their Functions