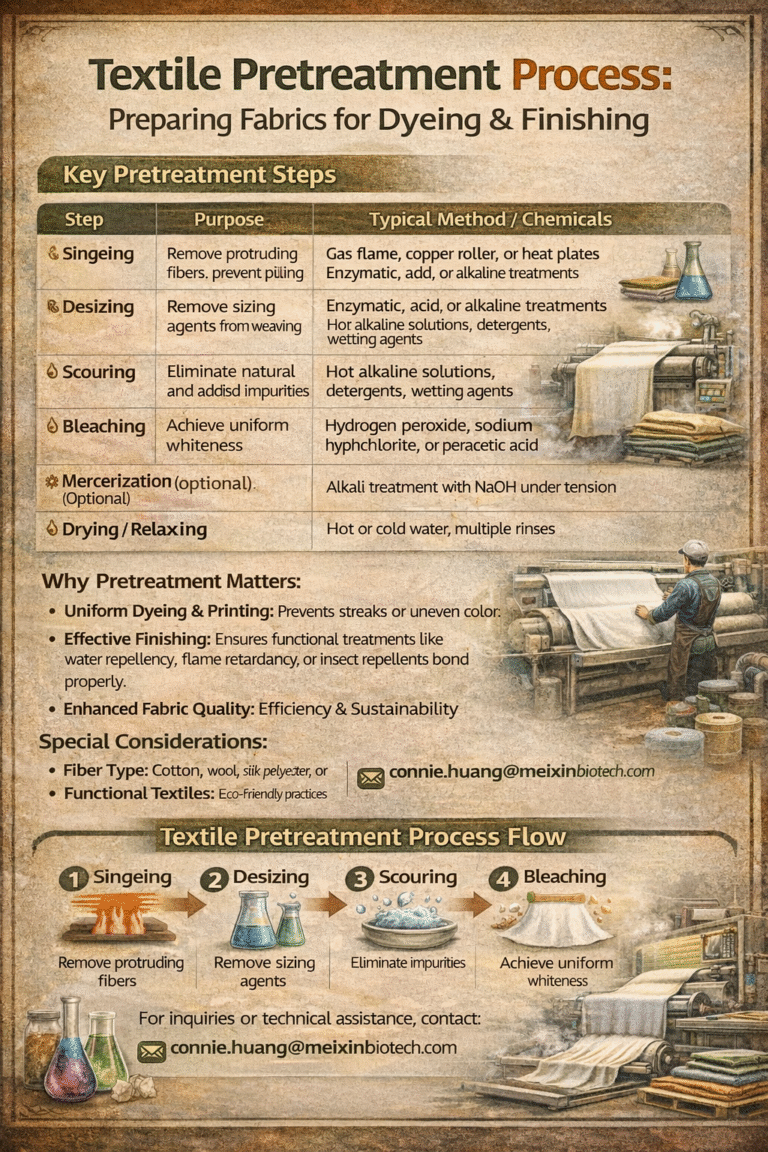
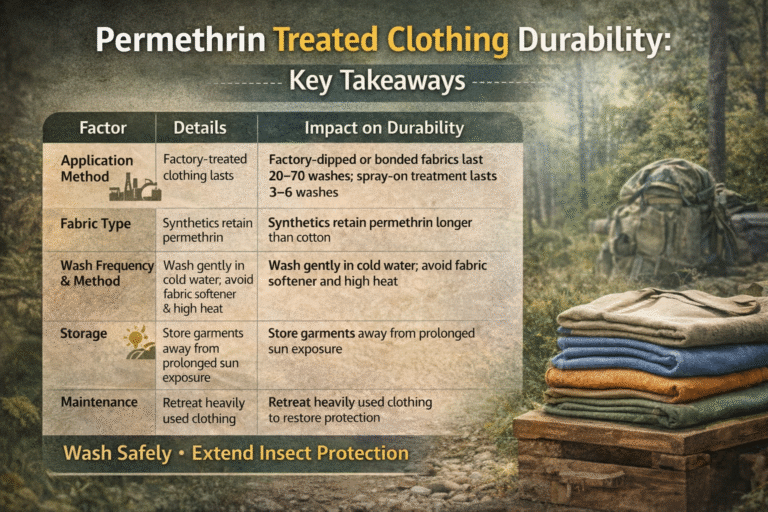
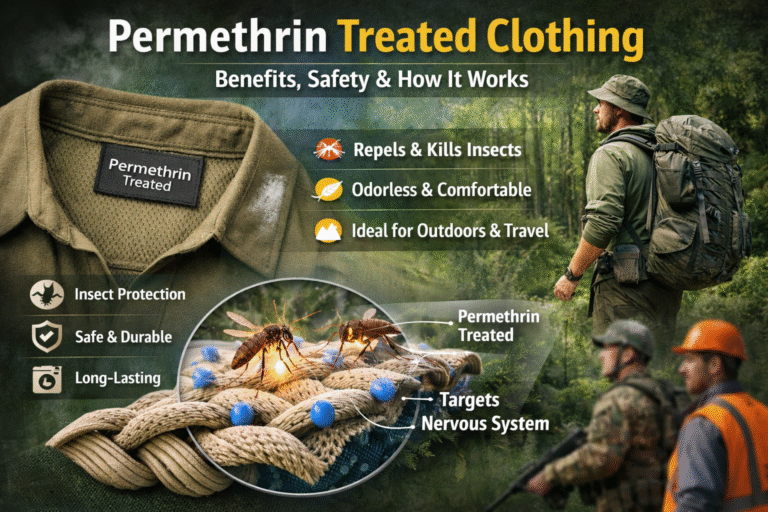
Color is one of the most powerful elements in textiles. From vibrant dresses to subtle home furnishings, dyes bring fabrics to life. Behind these colors lies the science of chemistry. Textile dyes can broadly be classified into natural dyes and synthetic dyes, each with unique properties, benefits, and challenges. Understanding the chemistry of both helps the textile industry balance performance, cost, and sustainability.
Natural Dyes: The Original Chemistry of Color
Natural dyes are derived from plants, minerals, and insects. Historically, they were the only source of textile color until synthetic chemistry transformed the industry in the 19th century.
Common Sources of Natural Dyes:
- Indigo (from indigo plants) → Blue shades
- Madder root → Red tones
- Turmeric or saffron → Yellow hues
- Cochineal insects → Deep crimson
Chemistry of Natural Dyes:
Most natural dyes are organic compounds like indigoids, anthraquinones, tannins, and flavonoids. They bond weakly to fibers, so mordants (such as alum, iron, or copper salts) are often needed to improve fixation and color fastness.
Advantages:
- Eco-friendly, biodegradable, and renewable
- Unique, earthy color tones
- Cultural and historical significance
Limitations:
- Poor color fastness without mordants
- Limited color range compared to synthetics
- Labor-intensive and costly at large scale
Synthetic Dyes: The Chemistry of Modern Textiles
The discovery of mauveine by William Henry Perkin in 1856 marked the birth of synthetic dyes. Today, synthetic dyes dominate the textile industry due to their versatility and efficiency.
Types of Synthetic Dyes Based on Chemistry:
- Azo dyes (contain –N=N– groups): Bright reds, yellows, oranges
- Anthraquinone dyes: Deep blues and greens
- Sulfur dyes: Dark colors like navy and black
- Reactive dyes: Form covalent bonds with fibers for excellent fastness
- Disperse dyes: Used for hydrophobic fibers like polyester
Advantages:
- Wide color palette with vibrant, uniform shades
- Better wash, light, and rub fastness
- Scalable for industrial use
- Lower production costs compared to natural dyes
Limitations:
- Environmental concerns due to non-biodegradability
- Use of toxic chemicals in some dye classes
- High water and energy consumption during processing
Natural vs. Synthetic Dyes: A Comparison
| Feature | Natural Dyes | Synthetic Dyes |
|---|---|---|
| Source | Plants, insects, minerals | Petrochemicals, synthetic compounds |
| Color Range | Limited, muted tones | Vast, vibrant spectrum |
| Color Fastness | Low without mordants | High with advanced chemistry |
| Eco-Friendliness | High (renewable, biodegradable) | Lower (pollution, waste issues) |
| Scalability | Small-scale, artisanal | Mass production |


The Future of Textile Dyes: Toward Sustainable Chemistry
The textile industry is exploring ways to combine the benefits of both natural and synthetic dyes. Innovations include:
- Bio-based dyes: Produced using biotechnology and microorganisms
- Eco-friendly mordants: Reducing heavy metal use in natural dyeing
- Low-impact synthetic dyes: Designed to minimize water and chemical waste
These efforts aim to balance vibrant colors, durability, and environmental responsibility.
Conclusion
Chemistry is at the heart of textile dyeing, from the ancient art of natural dyes to the precision of synthetic ones. While synthetic dyes dominate for their performance and cost-efficiency, natural dyes are regaining attention in the sustainability movement. The future of textile coloration lies in green chemistry innovations that merge durability with eco-friendliness.
For more insights into sustainable chemistry applications in textiles, feel free to contact Meixin Biotech at connie.huang@meixinbiotech.com.